New treatment procedures and new medications will be tested in clinical studies. What should you pay attention to before participating?
The essentials in summary:
- Clinical studies are required by law to test new medications, treatment methods, or medical devices to see if they are effective, safe, and tolerable. They initially occur with healthy volunteer guinea pigs.
- Only when the tolerability and effectiveness of new drugs or methods are researched in healthy volunteers will their effectiveness also be examined in sick people.
- Before participating in such a study, please inform yourself in detail about the conditions of the study and what obligations you assume by your participation.
What are clinical trials?
Before medicines are approved, their efficacy and safety must be proven in clinical studies. This will test whether a new medicine is more effective than a dummy medicine, also known as a placebo. New treatment methods are also being tested to see if they are better than the current standard of care.
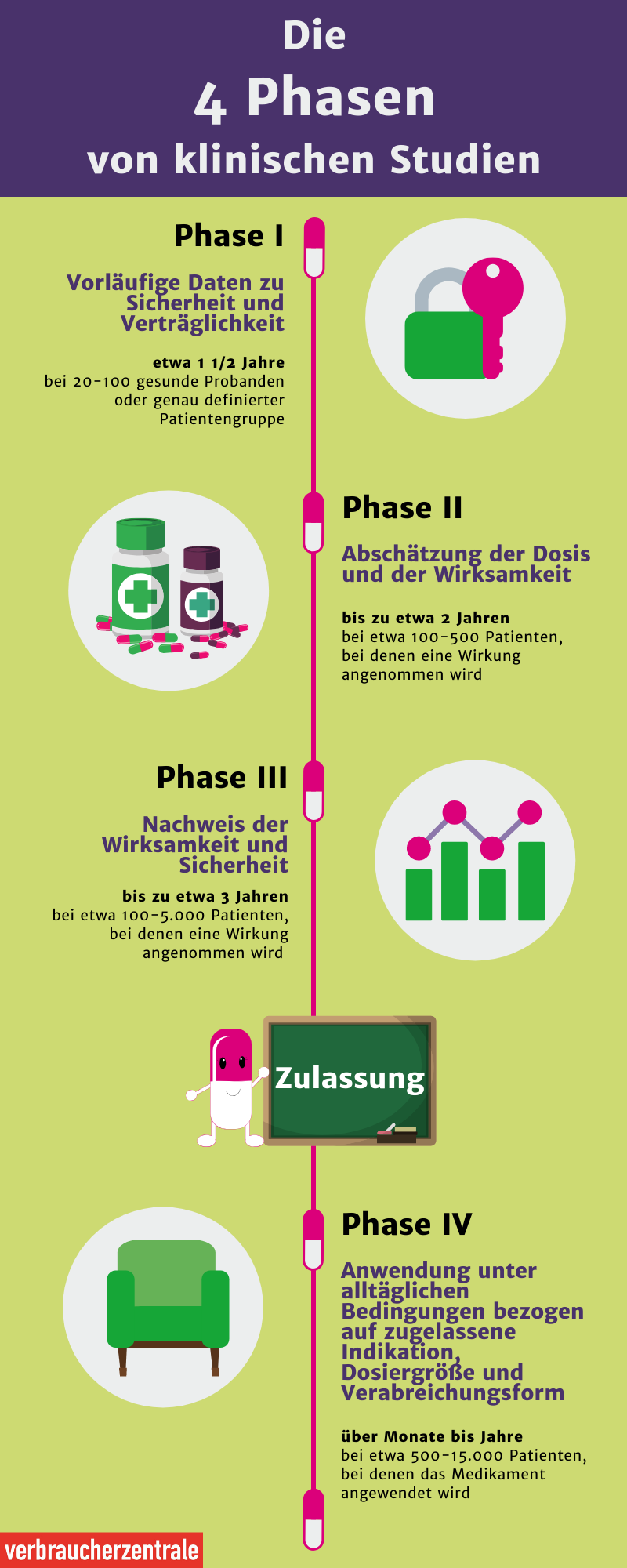
Before new drugs or medical treatments and examination procedures are used on humans, they are tested in animal and cell experiments. If the experiments are successful, the new method will be tested in human clinical studies.
Therefore, the development of a vaccine against Corona took some time. Compared to other vaccines, the SARS-CoV-2 vaccine was developed very quickly and approved early. While clinical phases usually last several years, this one lasted just under a year.
The way a clinical study is carried out, also known as study design, determines the quality of the results.
Studies carried out in the following way have the greatest significance:
- placebo controlled, in which the active ingredient and a simulated drug were compared,
- Randomized, meaning the forms of therapy you want to compare are randomly assigned. All test subjects are therefore randomly assigned to either the treatment group or the control group.
- Double-blind, which means that neither the doctor nor the subject knows which participant is in the treatment group or the control group that receives only the placebo.
It is a legal requirement that an ethics committee approve any controlled study testing a new medicine or treatment. The ethics committee must assess whether the benefits of the study outweigh any possible risks.
What happens to clinical trial data?
If phase III trials are successful, drug manufacturers will be able to apply for approval of the drug. Before the medicine can be prescribed by a doctor, regulatory authorities check the results of clinical studies.
approval authorities are
- the Federal Institute for Medicines and Medical Devices (BfArM)
- the Paul Ehrlich Institute for Vaccines and Biomedical Medicines (P.E.I.) It is
- in Europe, the European Medicines Agency (European Medicines Agency EMA)
As with any other treatment, the doctor draws up documents. It contains all information about the disease, treatment measures and test results. Unlike usual, in a study some data must be passed on.
This transfer is encrypted. Only certain institutions are allowed to view this data. This includes the client, the monitoring authority, and the federal authority that approved the study. Before participating in a study, you must agree that your data will be shared. The data will continue to be used even if you terminate the study prematurely.
Current data protection regulations apply to data storage. As a rule, they are kept for at least ten years. Everyone involved in the study is subject to confidentiality, such as doctors and nursing staff. You may not transmit your data to unrelated third parties.
What are the advantages or disadvantages of participating in clinical trials?
As a participant, you can receive treatment using procedures that are not yet available to the public. You will also receive particularly intensive medical care and monitoring. Depending on the study, you will often receive financial compensation based on the time spent.
However, previously unknown dangers may also arise:
- New methods may be less effective or even harmful compared to previous methods.
- Patients assigned to the control group receive a conventional drug or a dummy preparation.
- Furthermore, strict medical supervision may place a burden on you and restrictions. These include, for example, specific dates or documentation requirements.